Chris Allen


"Your Digital Trail for Safer Devices"

QSIG (Quality Signal)
Project Overview
The project involves designing a desktop app that streamlines the process of reporting BSC signals.
Responsibilities:
-
UX Research (client interviews)
-
UX Design (user flow and usability testing)
-
UI Design (high fidelity mockups)
Role: Lead Designer
Length of project: 6 weeks
Company: Boston Scientific
DISCOVERY
Project Brief
Boston Scientific is a global medical technology company that creates and sells medical devices. In a recent audit, the FDA found that the company did not have a reliable system for tracking and recording signals. Because of this, Boston Scientific must quickly build a solution to meet FDA compliance requirements before the next upcoming audit.
Research
The product owner and I spent two weeks interviewing and recording several internal BSC teams' feedback to understand the whos, whats, and whys of this project.
%20-%20t.jpg)
%20-%20Define%20-%20Future%20State.jpg)
Research Takeaways
Currently, BSC complies with FDA regulations by reporting adverse events through their Medical Device Reporting (MDR) system Socrates; however, the internal process for capturing and documenting signals related to defective equipment is flawed.
-
Signals are pieces of safety information or alerts that may suggest a medical device has a defect, risk, or unexpected effect. Tracking these signals helps companies detect problems early and take corrective action.
-
BSC has 19 manufacturing sites globally, and each site uses different processes and tools to record signals.
Personas

-
Process Owner: Primary user that will be recording signals and providing their disposition rationale.

-
Auditor: user who will be responsible for conducting audits. The user needs an easy-to-use tool that can quickly search and review large amounts of historical signal data for all BSC sites.

-
Admin: System admins will be responsible for setting permission levels within the app.
The Problem
BSC lacks a centralized system for recording signals or documenting disposition decisions for defective medical devices. Each manufacturing site uses its own tools and workflows, resulting in:
-
Inconsistent reporting practices
-
Limited visibility across the organization
-
Increased risk of non-compliance with regulatory standards
This fragmentation was exposed during a recent FDA audit, where the absence of a unified reporting system led to a violation and the potential for significant penalties.
The Solution
There is a clear need for a centralized, user-friendly platform that enables all Boston Scientific sites to:
-
Log and track defective device signals consistently
-
Document disposition decisions in a standardized format
-
Integrate seamlessly with the MDR framework for FDA reporting
-
Provide real-time visibility and analytics across global operations
By designing a cohesive digital solution, we can reduce compliance risks, improve operational efficiency, and ultimately enhance patient safety.
IDEATION
Defining User Flows
I collaborated with another designer in Miro to craft out the site map and the user flows for each user type.
%20-%20Define%20-%20User%20Flows_edited.jpg)
.jpg)
PROTOTYPE
Wireframing under time constraints
I used preexisting components in our Figma design system to create project components. I began by creating molecules and organisms to quickly craft mid-fidelity pages.
-
Atoms
-
Molecules

-
Organisms

-
Mid-fidelity wireframes

First Design walkthrough
I prioritized sharing mid-fidelity wireframes early to validate structure and usability with stakeholders. Their feedback helped shape the component system, which I then refined into high-fidelity designs using auto layout for responsiveness and consistency.
-
Established Project Components

-
High-fidelity Wireframes

ITERATING
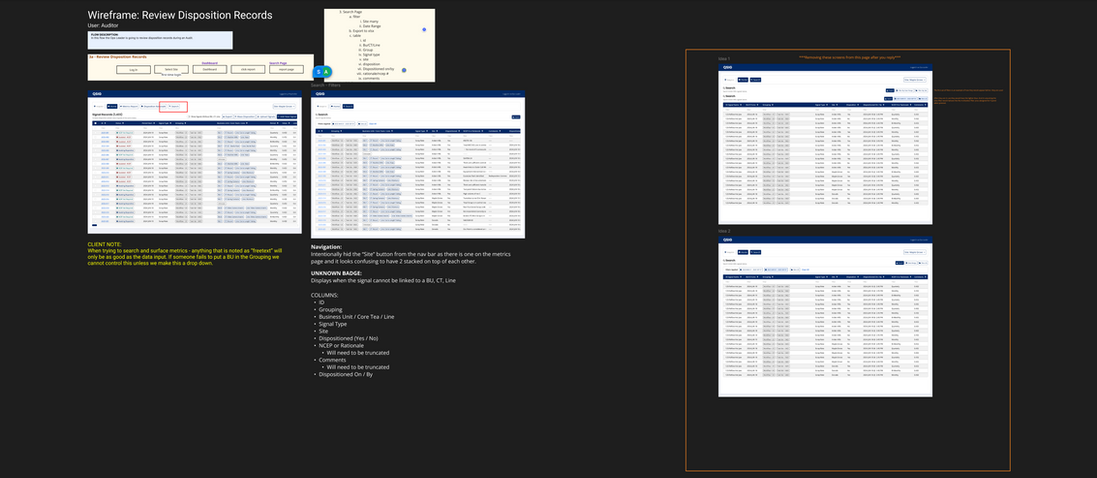
Client Feedback
I led 2 more design walkthroughs with the client before locking in the final versions. Given the data-heavy nature of the app, the feedback focused largely on which data should be prioritized and how labels should align with how users think. During those sessions, clients pinpointed the information they needed to see in table views without having to scroll horizontally, suggested additional metrics to include, and proposed renaming labels so that terms matched their familiar workflows.
Dashboard Iterations
-
Added mass dispositioning button/multi-select column
-
Added mass dispositioning instructive text
-
Renamed "Signal Name" column
-
Renamed Status badges
-
Deleted "Disposition" column
-
Changed date format for "period start" column
-
Updated "period" column timeframes
-
Updated grouping format to pills
-
Added 3 more columns
-
Reordered the columns by importance
-
Added signal count
-
Relocated site selector
-
Added toggle for unique identifiers
Signal Page Iterations
-
Relabel Signal Name Field
-
Removed Site Selector
-
Added grouping sample text
-
Reordered form fields
Before Iterations

After Iterations

After receiving positive client feedback, the designs were finalized and handed off to the dev team. I added detailed dev notes in Figma to support a smooth build process and minimize ambiguity. With development underway, Boston Scientific is on track to meet the FDA deadline with a tool that’s both user-centered and audit-ready.
Conclusion
The QSIG app now offers Boston Scientific a unified, streamlined source for all 19 sites to generate signals, capture disposition rationales, and enable auditors to transparently track signal activity across every BSC location. By closing a critical standardization gap, the app helps ensure FDA compliance—and, above all, delivers a tool that meaningfully supports patient health, BSC’s top priority.
The dashboard features all the metadata about submitted signals for the specified site.

The signal upload page allows the user to mass upload signals via the Excel template provided.

The signal/disposition form allows users to provide details about the signal and what actions need to be taken.

The metrics history page allows users to see high-level metrics about signals for the specified sites.

The disposition rationale history page allows users to see high-level metrics about dispositioned signals for the specified sites.

The business hierarchy page allows users to create a customizable structure to help find signals.

The data source page allows users to upload signals from a UDP database.

Project Impact
-
BSC Compliance Confidence: The app supports FDA audit compliance ahead of the deadline.
-
BSC Process Standardization: This app has closed the standardization gap for signal workflows, and now serves as a blueprint for identifying and resolving similar gaps elsewhere.
-
Client Satisfaction & Usability: Stakeholders approved the final design with minimal revisions, recognizing that the new tool significantly improves on past workflows—making tasks faster, more intuitive, and less error-prone.